Application for import license of COVID-19 pharmaceutical raw materials 3 months reduced
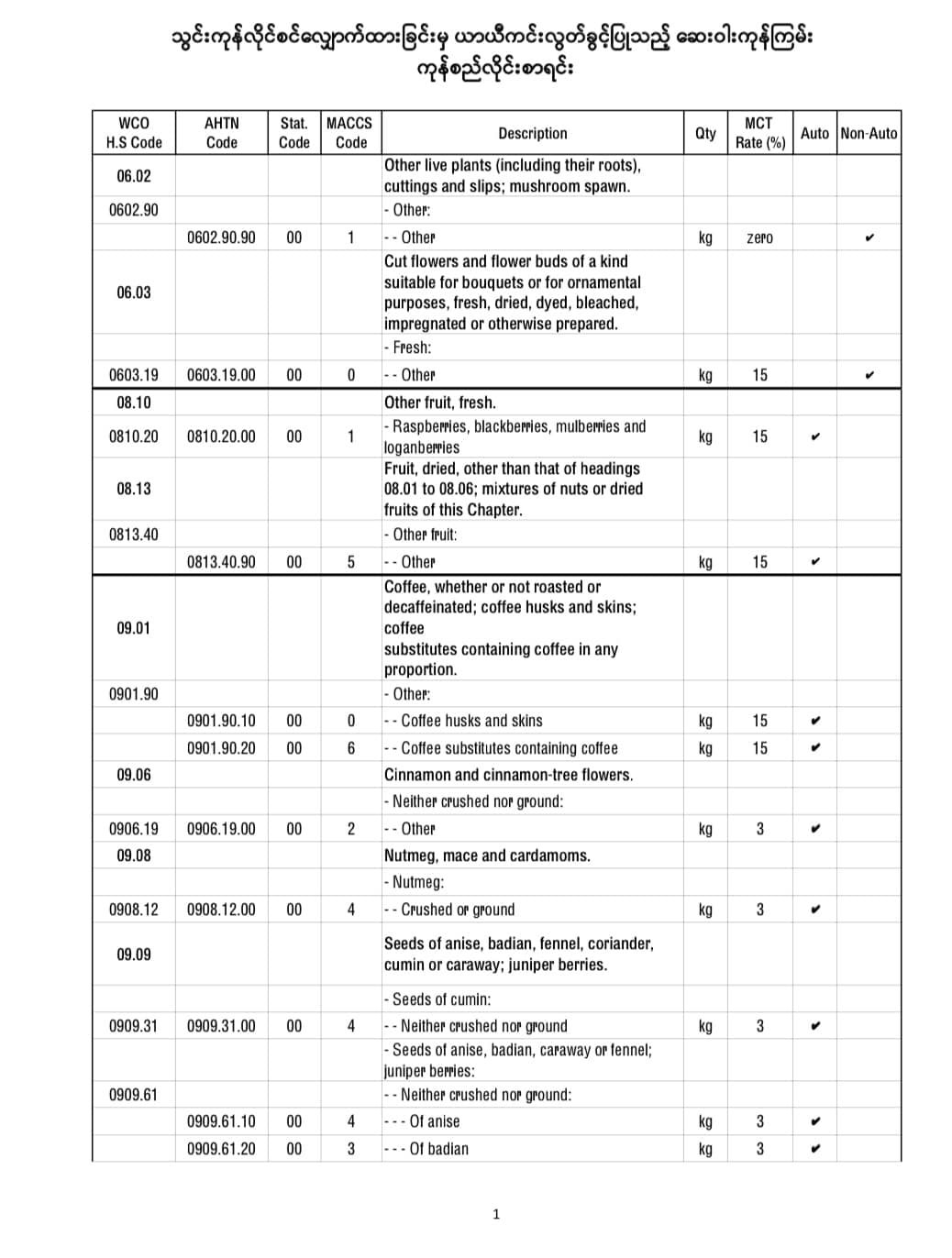
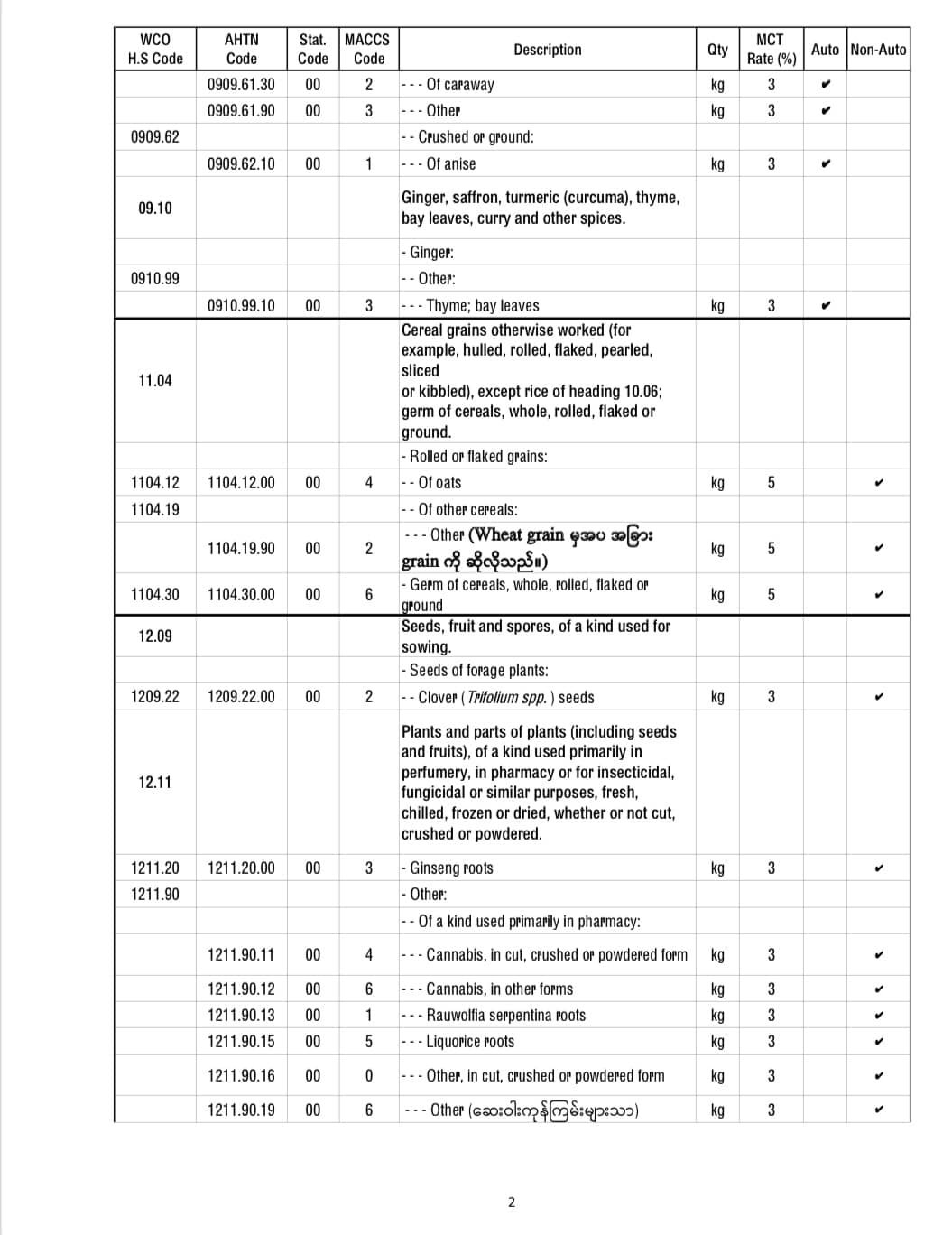
It is announced that the temporary exemption of application for import license of COVID-19 pharmaceutical raw materials 3 months reduced.
Among the products used as raw materials for pharmaceutical products to support the prevention and control of COVID-19 infection, the 92 freights were allowed to import temporarily for 3 months starting from 2021, without needing to apply for an import license.
Here are some of the temporary import license-free products from applying for import licenses.
Photo Source https://bit.ly/3zD44vI
Check the following link for more information.